M K Verma Solutions for Chapter: Chemical Bonding and Molecular Structure, Exercise 1: TOPICWISE QUESTIONS
M K Verma Chemistry Solutions for Exercise - M K Verma Solutions for Chapter: Chemical Bonding and Molecular Structure, Exercise 1: TOPICWISE QUESTIONS
Attempt the free practice questions on Chapter 1: Chemical Bonding and Molecular Structure, Exercise 1: TOPICWISE QUESTIONS with hints and solutions to strengthen your understanding. Practice Book for MHT-CET Chemistry (Inorganic Chemistry 1) solutions are prepared by Experienced Embibe Experts.
Questions from M K Verma Solutions for Chapter: Chemical Bonding and Molecular Structure, Exercise 1: TOPICWISE QUESTIONS with Hints & Solutions
In which of the following molecules, the central atom expands its covalency by excitation but not its octet?
(a)
(b)
(c)
(d)
Assuming the Hund's rule violated, the bond order and magnetic nature of the diatomic molecule of is:
The diamagnetic species among the following is/are:
. .
. .
Among the following pairs of molecules, the strongest intermolecular hydrogen bond is present between:
What is true about the given molecules?
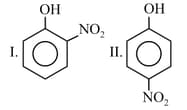
Which of the three molecules, and , has the minimum ratio of (where, is the magnitude of a bond angle and is the number of lone pairs of electrons in the molecule)?
The ratio of coordinate bonds, covalent bonds and hydrogen bonds in is:
Considering the following three species, identify the incorrect order in the given options.
