Academic Experts Solutions for Chapter: Chemical Kinetics & Nuclear Chemistry, Exercise 2: KVPY PROBLEMS (PREVIOUS YEARS)
Academic Experts Chemistry Solutions for Exercise - Academic Experts Solutions for Chapter: Chemical Kinetics & Nuclear Chemistry, Exercise 2: KVPY PROBLEMS (PREVIOUS YEARS)
Attempt the free practice questions on Chapter 2: Chemical Kinetics & Nuclear Chemistry, Exercise 2: KVPY PROBLEMS (PREVIOUS YEARS) with hints and solutions to strengthen your understanding. Practice Book for KVPY Aptitude Test - Stream SX Chemistry solutions are prepared by Experienced Embibe Experts.
Questions from Academic Experts Solutions for Chapter: Chemical Kinetics & Nuclear Chemistry, Exercise 2: KVPY PROBLEMS (PREVIOUS YEARS) with Hints & Solutions
The decay profiles of three radioactive species and are given below:
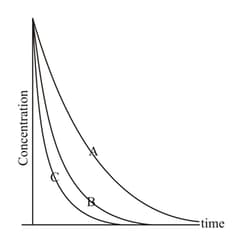
These profiles imply that the decay constants and follow the order:
For a zero-order reaction with rate constant , the slope of the plot of reactant concentration against time is:
The Arrhenius plots of two reactions, and are shown graphically:
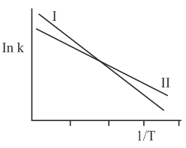
The graph suggests that:
The rate constant of a chemical reaction at a very high temperature will approach:
It takes for a first-order reaction to go to completion. The total time required for the same reaction to reach completion will be:
In the radioactive disintegration series involving and decay, the total number of and particles emitted are:
has a half-life of years with respect to radioactive decay. The decay follows two parallel paths:
and . If the percentage of the two daughter nuclides are and , respectively, the decay constant (in ) for path is closest to:
Consider the following reversible first-order reaction of at an initial concentration . The values of the rate constants are and .
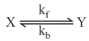
A plot of concentration of and as function of time is:
