Lawrie Ryan and Roger Norris Solutions for Chapter: Benzene and its Compounds, Exercise 11: EXAM-STYLE QUESTIONS
Lawrie Ryan Chemistry Solutions for Exercise - Lawrie Ryan and Roger Norris Solutions for Chapter: Benzene and its Compounds, Exercise 11: EXAM-STYLE QUESTIONS
Attempt the free practice questions on Chapter 25: Benzene and its Compounds, Exercise 11: EXAM-STYLE QUESTIONS with hints and solutions to strengthen your understanding. Chemistry for Cambridge International AS & A Level Coursebook with Digital Access (2 Years) solutions are prepared by Experienced Embibe Experts.
Questions from Lawrie Ryan and Roger Norris Solutions for Chapter: Benzene and its Compounds, Exercise 11: EXAM-STYLE QUESTIONS with Hints & Solutions
Benzene can be nitrated to give nitrobenzene. Name the mechanism for this reaction.
Benzene can be nitrated to give nitrobenzene. The species attacking benzene in the reaction is . How is generated in the reaction mixture? Name the substances used and give a chemical reaction leading to the formation of .
Benzene can be nitrated to give nitrobenzene. Give a suitable temperature for this reaction.
Benzene can be nitrated to give nitrobenzene. Use curly arrows to draw the mechanism of how benzene reacts with to produce nitrobenzene.
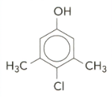
Benzene can be nitrated to give nitrobenzene. The structure of a common household substance is given below:
Give the molecular formula of the compound.
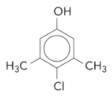
Benzene can be nitrated to give nitrobenzene. The structure of a common household substance is given below:
When bromine water is added to a solution of the compound, it is decolourised. Suggest two structures for the product of the reaction.
Describe the bonding in benzene. Include a description of the model used for the arrangement of electrons in the molecules.
Cyclohexene decolourises bromine water whereas benzene has no effect on bromine water. Explain the difference in reactivity towards bromine water.