Lawrie Ryan and Roger Norris Solutions for Chapter: Electrons in Atoms, Exercise 15: EXAM-STYLE QUESTIONS
Lawrie Ryan Chemistry Solutions for Exercise - Lawrie Ryan and Roger Norris Solutions for Chapter: Electrons in Atoms, Exercise 15: EXAM-STYLE QUESTIONS
Attempt the free practice questions on Chapter 2: Electrons in Atoms, Exercise 15: EXAM-STYLE QUESTIONS with hints and solutions to strengthen your understanding. Chemistry for Cambridge International AS & A Level Coursebook with Digital Access (2 Years) solutions are prepared by Experienced Embibe Experts.
Questions from Lawrie Ryan and Roger Norris Solutions for Chapter: Electrons in Atoms, Exercise 15: EXAM-STYLE QUESTIONS with Hints & Solutions
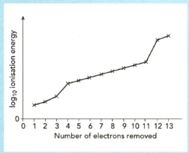
The sketch graph shows the successive ionization energies of aluminium.
Define the term first ionization energy.
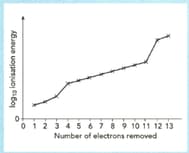
The sketch graph shows the successive ionization energies of aluminium.
Explain how the graph provides evidence for the existence of three electrons shells in an aluminium atom.
Write an equation, including state symbols, to represent the ionization energy aluminium.
Write the electronic configuration of an aluminium ion, using notation.
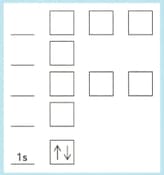
Copy and complete the diagram below for the electrons in phosphorus by:
Showing how the electrons are arranged.
State which ionization energies are represented by the equations blow.
The graph shows a sketch of (ionization energy) against number of electrons removed for magnesium.
Use this graph to answer the following questions.
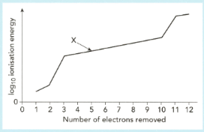
Construct the equation for the ionization energy marked (the ionization energy).
Explain the shape of the graph you have drawn for (ionization energy) for Chlorine varies when plotted against number of electron removed.