EXAM-STYLE QUESTIONS
Lawrie Ryan Chemistry Solutions for Exercise - EXAM-STYLE QUESTIONS
Simple step-by-step solutions to EXAM-STYLE QUESTIONS questions of Lattice Energy from Chemistry for Cambridge International AS & A Level Coursebook with Digital Access (2 Years). Also get 3D topic explainers, cheat sheets, and unlimited doubts solving on EMBIBE.
Questions from EXAM-STYLE QUESTIONS with Hints & Solutions
Use ideas about ion polarisation to explain why barium carbonate is more stable to thermal decomposition than magnesium carbonate.
The diagram shows the enthalpy changes when sodium chloride is dissolved in water.
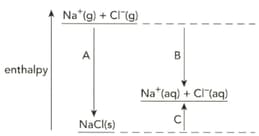
Define the term enthalpy change of solution.
The diagram shows the enthalpy changes when sodium chloride is dissolved in water.
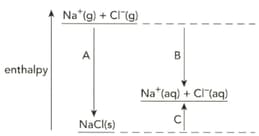
Define the term enthalpy change of hydration.
The diagram shows the enthalpy changes when sodium chloride is dissolved in water.
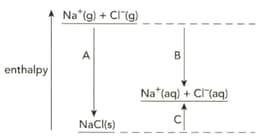
Write symbol equation that describe the enthalpy change of solution of sodium chloride.
The diagram shows the enthalpy changes when sodium chloride is dissolved in water.
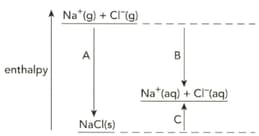
Write symbol equation that describe the enthalpy change of hydration of the chloride ion.
The diagram shows the enthalpy changes when sodium chloride is dissolved in water.
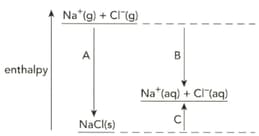
Name the enthalpy changes labelled A, B and C.
Draw the water molecules around magnesium ions and sulfate ions in a solution of magnesium sulfate.
Explain, in terms of differences of lattice energies and enthalpy changes of hydration, why magnesium sulfate is more soluble in water than calcium sulfate.
