RoseMarie Gallagher and Paul Ingram Solutions for Chapter: Atoms Combining, Exercise 14: Checkup on Chapter 4
RoseMarie Gallagher Chemistry Solutions for Exercise - RoseMarie Gallagher and Paul Ingram Solutions for Chapter: Atoms Combining, Exercise 14: Checkup on Chapter 4
Attempt the free practice questions on Chapter 4: Atoms Combining, Exercise 14: Checkup on Chapter 4 with hints and solutions to strengthen your understanding. Complete Chemistry for Cambridge IGCSE® Second Edition solutions are prepared by Experienced Embibe Experts.
Questions from RoseMarie Gallagher and Paul Ingram Solutions for Chapter: Atoms Combining, Exercise 14: Checkup on Chapter 4 with Hints & Solutions
The compound zinc sulphide has a structure like this:
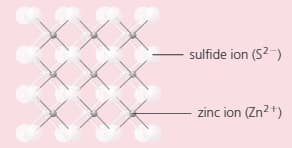
Which does the diagram represent: a giant structure, or a molecular structure?
The compound zinc sulphide has a structure like this:
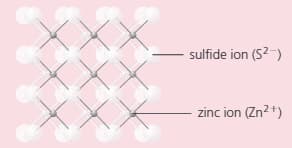
Which type of bonding does zinc sulphide have?
The compound zinc sulphide has a structure like this:
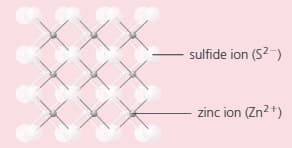
Look carefully at the structure. How many:
i) sulphur ions are joined to each zinc ion?
ii) zinc ions are joined to each sulphur ion?
The compound zinc sulphide has a structure like this:
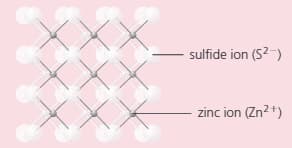
i) Deduce the formula of zinc sulphide.
ii) Is this formula consistent with the charges on the two ions? Explain your answer.
The compound zinc sulphide has a structure like this:
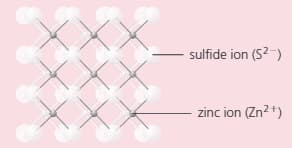
Name another metal and non-metal that will form a compound with a similar formula as that of zinc sulphide.
The properties of metals can be explained by the structure and bonding within the metal lattice. Describe the bonding in metals.
The properties of metals can be explained by the structure and bonding within the metal lattice. Use the bonding to explain why metals are good conductors of electricity.
The properties of metals can be explained by the structure and bonding within the metal lattice. Use the bonding to explain why metals are malleable and flexible.
