RoseMarie Gallagher and Paul Ingram Solutions for Chapter: Separating Substances, Exercise 6: Checkup on Chapter 2
RoseMarie Gallagher Chemistry Solutions for Exercise - RoseMarie Gallagher and Paul Ingram Solutions for Chapter: Separating Substances, Exercise 6: Checkup on Chapter 2
Attempt the free practice questions on Chapter 2: Separating Substances, Exercise 6: Checkup on Chapter 2 with hints and solutions to strengthen your understanding. Complete Chemistry for Cambridge IGCSE® Second Edition solutions are prepared by Experienced Embibe Experts.
Questions from RoseMarie Gallagher and Paul Ingram Solutions for Chapter: Separating Substances, Exercise 6: Checkup on Chapter 2 with Hints & Solutions
A mixture of salt and sugar has to be separated, using the solvent ethanol. Draw a diagram of the apparatus used in the experiment of obtaining sugar crystals from the sugar solution, without losing the ethanol.
In a chromatography experiment, eight coloured substances were spotted on a piece of filter paper. Three were the basic coloured red, blue and yellow. The other was unknown substances, labelled A-E. This shows the resulting chromatogram :
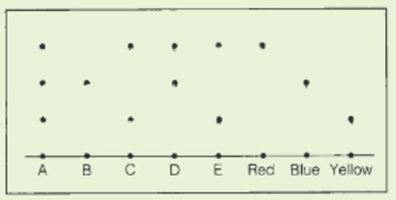
Which one of substance A-E contains only one basic colour?
In a chromatography experiment, eight coloured substances were spotted onto a piece of filter paper. Three were the basic coloured red, blue and yellow. The other were unknown substances, labelled A-E. This shows the resulting chromatogram :

Which contains all three basic colours?
In a chromatography experiment, eight coloured substances were spotted on a piece of filter paper. Three were the basic coloured red, blue and yellow. The other was unknown substances, labelled A-E. This shows the resulting chromatogram :

The solvent was propanone. Which of the three basic colours are the most soluble in propanone?
The diagram below shows a chromatogram for a mixture of amino acids.
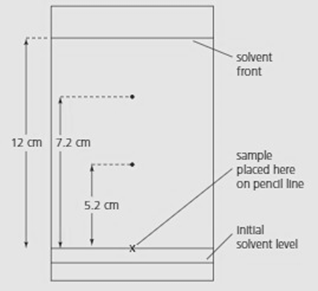
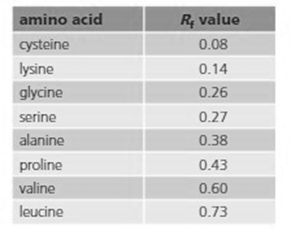
The solvent was a mixture of water, butanol and ethanoic acid. Using the table of values given, identify the two amino acids.
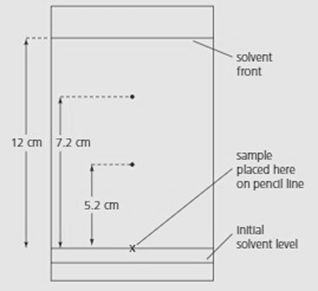
The diagram below shows a chromatogram for a mixture of amino acids.


The solvent was a mixture of water, butanol and ethanoic acid. Which of them is less soluble in the solvent?
The diagram below shows a chromatogram for a mixture of amino acids.
| Amino acid | value |
| Cysteine | 0.08 |
| Lysine | 0.14 |
| Glycine | 0.26 |
| Serine | 0.27 |
| Alanine | 0.38 |
| Proline | 0.43 |
| Valine | 0.60 |
| Leucine | 0.73 |
The solvent was a mixture of water, butanol and ethanoic acid. How will the values change if the solvent travels only cm?
You have three colourless solutions. Each contains an amino acid you must identify. Explain how to do this using chromatography. Use the term and locating agent in your answer, and show that you understand what they mean.
