RoseMarie Gallagher and Paul Ingram Solutions for Chapter: The Speed of a Reaction, Exercise 11: Checkup on Chapter 10
RoseMarie Gallagher Chemistry Solutions for Exercise - RoseMarie Gallagher and Paul Ingram Solutions for Chapter: The Speed of a Reaction, Exercise 11: Checkup on Chapter 10
Attempt the free practice questions on Chapter 10: The Speed of a Reaction, Exercise 11: Checkup on Chapter 10 with hints and solutions to strengthen your understanding. Complete Chemistry for Cambridge IGCSE® Second Edition solutions are prepared by Experienced Embibe Experts.
Questions from RoseMarie Gallagher and Paul Ingram Solutions for Chapter: The Speed of a Reaction, Exercise 11: Checkup on Chapter 10 with Hints & Solutions
Zinc and iodine solution react like this:
The rate of reaction can be followed by measuring the mass of zinc metal at regular intervals, until all the iodine has been used up.
Sketch a graph showing the mass of zinc on the axis, and time on the axis.
Zinc and iodine solution react like this:
The rate of reaction can be followed by measuring the mass of zinc metal at regular intervals, until all the iodine has been used up.
How will the graph change if the temperature of the iodine solution is increased by ?
Zinc and iodine solution react like this:
The rate of reaction can be followed by measuring the mass of zinc metal at regular intervals, until all the iodine has been used up.
How will the graph between concentration of zinc and time changes, if the temperature of the iodine solution is increased by ? Explain your answer using the idea of collisions between particles.
Some pondweed is placed as shown:
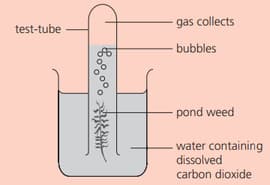
Name the gas that collects in the test tube.
Some pondweed is placed as shown:
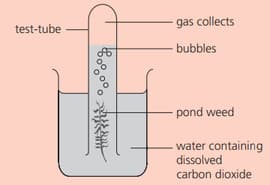
What is the products formed other than oxygen?
Some pondweed is placed as shown:
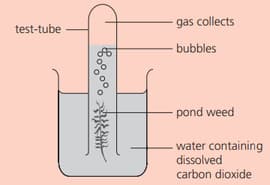
This experiment must be carried out in the light. Why?
Some pondweed is placed as shown:
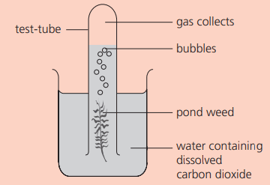
Using the apparatus above, suggest a method by which the rate of reaction could be found.
Some pondweed is placed as shown:
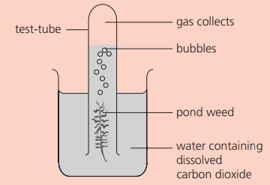
What would be the effect of bringing a lamp close to the beaker? Explain your answer.
