V. K. Sally and R. Goel Solutions for Chapter: Metals And Non-Metals, Exercise 3: EXERCISE 3C
V. K. Sally Chemistry Solutions for Exercise - V. K. Sally and R. Goel Solutions for Chapter: Metals And Non-Metals, Exercise 3: EXERCISE 3C
Attempt the practice questions on Chapter 3: Metals And Non-Metals, Exercise 3: EXERCISE 3C with hints and solutions to strengthen your understanding. Core Science Chemistry solutions are prepared by Experienced Embibe Experts.
Questions from V. K. Sally and R. Goel Solutions for Chapter: Metals And Non-Metals, Exercise 3: EXERCISE 3C with Hints & Solutions
A small piece of sodium is dropped into a beaker containing water. Which of the following observations are incorrect?
Which of the following reactions is not possible?
Although metals form basic oxides, which of the following metals form an amphoteric oxide?
Which of the following metals are obtained by the electrolysis of their chlorides in the molten state?
2 mL each of concentrated and a mixture of concentrated and concentrated in the ratio of 3 : 1 were taken in test tubes labelled as A, B and C. A small piece of metal was put in each test tube. No change occurred in test tubes A and B but the metal got dissolved in test tube C respectively. The metal could be
Sample pieces of five metals, A, B, C, D and E were added to the tabulated solutions separately. The results observed were as shown in the table below.
| Metal | Solution | ||||
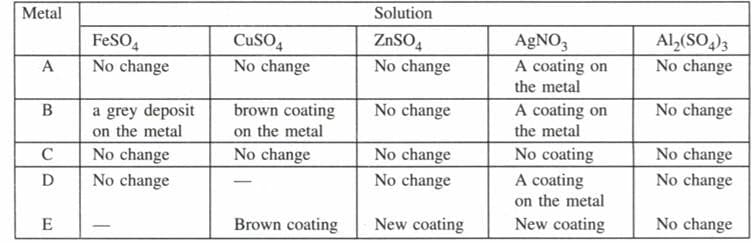
Based on the observations recorded in the table answer following questions
(i) Which is the most reactive metal?
(ii) Which is the least reactive metal?
(iii) What would be observe if metal D were added to the solution of copper (II) sulphate?
(iv) What would be observed if metal E were added to a solution of iron (II) sulphate?
(v) Arrange the metals A, B, C, D and E in the order of their decreasing reactivity.
An element A burns with a golden flame in air. It reacts with another element B, atomic number 17 to give a product C. An aqueous solution of the product C on electrolysis gives a compound D and liberates hydrogen. Identify A, B, C and D. Also write down the equations for the reactions involved.
What happens when an iron strip is put into separate beakers containing aqueous solution of copper sulphate and zinc sulphate? Where is iron placed in the activity series with respect to copper? Describe the steps involved in the extraction of zinc from its sulphide and carbonate ores. Support your answer with balanced chemical equation for the chemical reactions involved in the process.
