David Sang Solutions for Chapter: The Nuclear Atom, Exercise 4: End-of-chapter questions
David Sang Physics Solutions for Exercise - David Sang Solutions for Chapter: The Nuclear Atom, Exercise 4: End-of-chapter questions
Attempt the free practice questions on Chapter 22: The Nuclear Atom, Exercise 4: End-of-chapter questions with hints and solutions to strengthen your understanding. Cambridge IGCSE® Physics Coursebook Second Edition solutions are prepared by Experienced Embibe Experts.
Questions from David Sang Solutions for Chapter: The Nuclear Atom, Exercise 4: End-of-chapter questions with Hints & Solutions
The diagram represents a neutral lithium atom. All the particles in the atom are shown on the diagram. Use the diagram to help you answer the following question. What is the value of the proton number of this atom?
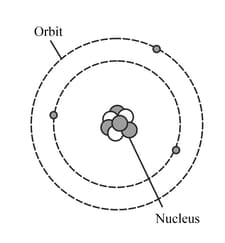
The diagram represents a neutral lithium atom. All the particles in the atom are shown on the diagram. Use the diagram to help you answer the following question. How many neutrons does the atom have?
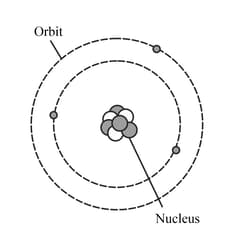
The diagram represents a neutral lithium atom. All the particles in the atom are shown on the diagram. Use the diagram to help you answer the following question. What is the value of the nucleon number of this atom?
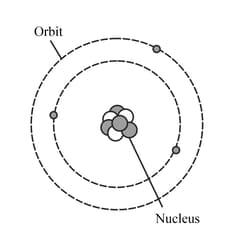
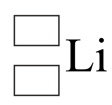
Copy the diagram below, replacing the boxes with appropriate numbers, to represent this atom of lithium in nuclide notation.
 .
.
The charges on the particles in an atom may be represented by or or ,
The masses of the particles in an atom may be represented by or or .
Using these choices, copy and complete the table.
| Particle | Charge | Mass |
| Electron | ||
| Neutron | ||
| Proton |
How many of these particles are there in a neutral atom of
Electrons.
How many of these particles are there in a neutral atom of .
neutrons
How many of these particles are there in an neutral atom of
protons
