Dr. Parul Srivastava Solutions for Chapter: Acids, Bases and Salts, Exercise 14: Formative Assessment
Dr. Parul Srivastava Chemistry Solutions for Exercise - Dr. Parul Srivastava Solutions for Chapter: Acids, Bases and Salts, Exercise 14: Formative Assessment
Attempt the practice questions on Chapter 2: Acids, Bases and Salts, Exercise 14: Formative Assessment with hints and solutions to strengthen your understanding. PRACHI SCIENCE CHEMISTRY CLASS-X solutions are prepared by Experienced Embibe Experts.
Questions from Dr. Parul Srivastava Solutions for Chapter: Acids, Bases and Salts, Exercise 14: Formative Assessment with Hints & Solutions
The values of distilled water, lemon juice, sodium bicarbonate were measured using paper. What is the correct decreasing order of values?
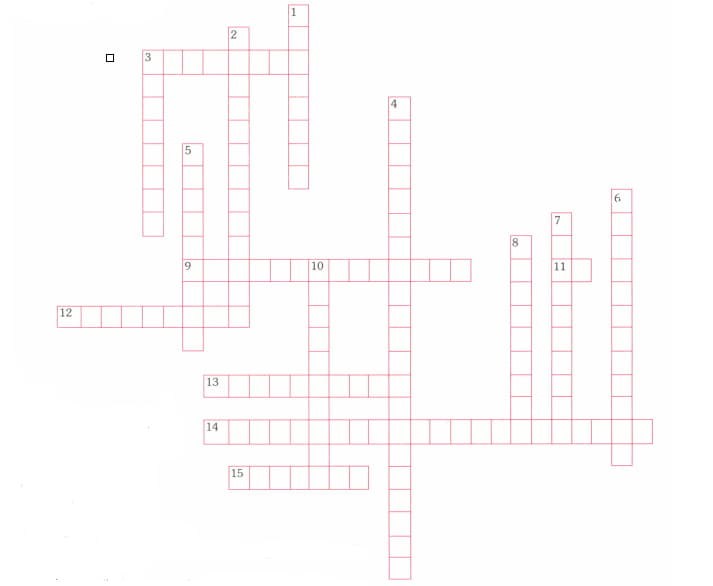
SOLVE THE PUZZLE.
Down:
1. The point at which the indicator changes colour.
2. A solution in which is less than .
3. A base that reacts with water to form the hydroxide ion and the conjugate acid of the base.
4. Two substances related by the loss or gain of a single hydrogen ion.
5. A substance that can accept a pair of electrons to form a covalent bond.
6. A water molecule that gain a hydrogen ion becomes a positively charged ion.
7. A substance that can act as both an acid and a base.
8. A substance that can donate a pair of electrons to form a covalent bond.
10. A base that completely dissociates into metal ions and hydroxide ion in aqueous solution.
Across:
3. An acid that is only slightly ionised in aqueous solution.
9. A solution in which is greater than .
11. The negative logarithm of the hydrogen-ion concentration.
12. The process of adding a known amount of solution of known concentration to determine the concentration of another solution.
14. A reaction in which an acid and a base reacts to produce salt and water.
15. A solution in which the pH remains relatively constant when small amounts of acid or base are added.
What is the colour of phenolphthalein in soap solution?
Which one of the following types of medicines is used for treating acidity?
Each colour is associated with a particular in
Phenolphthalein gives_____colour at < .
What will be the colour of acidic and basic solutions when dipped in blue litmus paper respectively?
Sodium hydroxide solution turns red litmus paper to
