Dr. S.N. DHAWAN, Dr. S.C.KHETERPAL and, P.N.KAPIL Solutions for Chapter: Organic Chemistry - Some Basic Principles and Techniques, Exercise 2: TEST YOUR GRIP
Dr. S.N. DHAWAN Chemistry Solutions for Exercise - Dr. S.N. DHAWAN, Dr. S.C.KHETERPAL and, P.N.KAPIL Solutions for Chapter: Organic Chemistry - Some Basic Principles and Techniques, Exercise 2: TEST YOUR GRIP
Attempt the practice questions on Chapter 12: Organic Chemistry - Some Basic Principles and Techniques, Exercise 2: TEST YOUR GRIP with hints and solutions to strengthen your understanding. Pradeep's New Course CHEMISTRY Vol.II solutions are prepared by Experienced Embibe Experts.
Questions from Dr. S.N. DHAWAN, Dr. S.C.KHETERPAL and, P.N.KAPIL Solutions for Chapter: Organic Chemistry - Some Basic Principles and Techniques, Exercise 2: TEST YOUR GRIP with Hints & Solutions
of an organic compound containing phosphorus gave of by usual analysis. Calculate the percentage of phosphorus in the organic compound.
The IUPAC name of 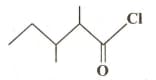 is
is
The IUPAC name of 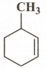 is
is
of organic compound on kjeldahlization liberates ammonia which consumes of . The percentage of nitrogen in the compound is
The kind of delocalisation involving -bond orbitals is called _____ . (resonance/hyperconjugation)
Among isomeric butenes, _____ is the most stable.(trans-2-butene/cis-2-butene)
The bond dissociation energy needed to form the benzyl radical from toluene is _____ (greater/lower) than the energy needed for the formation of methyl radical from methane in terms of lower or higher.
Liquids which decompose at or lower than their boiling points are purified by _____ under reduced pressure.
