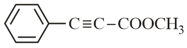Embibe Experts Solutions for Chapter: Chemical Bonding and Molecular Structure, Exercise 1: AIPMT - 25th July 2015
Embibe Experts Chemistry Solutions for Exercise - Embibe Experts Solutions for Chapter: Chemical Bonding and Molecular Structure, Exercise 1: AIPMT - 25th July 2015
Attempt the free practice questions on Chapter 4: Chemical Bonding and Molecular Structure, Exercise 1: AIPMT - 25th July 2015 with hints and solutions to strengthen your understanding. EMBIBE CHAPTER WISE PREVIOUS YEAR PAPERS FOR CHEMISTRY solutions are prepared by Experienced Embibe Experts.
Questions from Embibe Experts Solutions for Chapter: Chemical Bonding and Molecular Structure, Exercise 1: AIPMT - 25th July 2015 with Hints & Solutions
Amongst the following which one will have maximum 'lone pair - lone pair' electron repulsions?
Which amongst the following is incorrect statement?
Match List - I with List - II.
| List-I | List-II | ||
| (a) | (i) | Square pyramidal | |
| (b) | (ii) | Trigonal planar | |
| (c) | (iii) | Octahedral | |
| (d) | (iv) | Trigonal bipyramidal | |
Choose the correct answer from the options given below.
Which of the following molecules is non-polar in nature?
Match the compounds of Xe in column I with the molecular structure in column II.
| Column-I | Column-II | ||
| (a) | (p) | Square planar | |
| (b) | (q) | Linear | |
| (c) | (r) | Square pyramidal | |
| (d) | (s) | Pyramidal |
Identify the wrongly matched pair:
The potential energy curve for formation as a function of internuclear distance of the atoms is shown below.
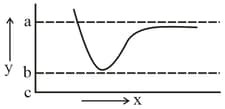
The bond energy of is :
How many (i) hybridised carbon atoms and (ii) bonds are present in the following compound?