Embibe Experts Solutions for Chapter: Hydrocarbons, Exercise 1: KVPY Aptitude Test - Stream SA 2020
Embibe Experts Chemistry Solutions for Exercise - Embibe Experts Solutions for Chapter: Hydrocarbons, Exercise 1: KVPY Aptitude Test - Stream SA 2020
Attempt the practice questions on Chapter 16: Hydrocarbons, Exercise 1: KVPY Aptitude Test - Stream SA 2020 with hints and solutions to strengthen your understanding. EMBIBE CHAPTER WISE PREVIOUS YEAR PAPERS FOR CHEMISTRY solutions are prepared by Experienced Embibe Experts.
Questions from Embibe Experts Solutions for Chapter: Hydrocarbons, Exercise 1: KVPY Aptitude Test - Stream SA 2020 with Hints & Solutions
Among the following, the most acidic compound is :
A hydrocarbon with molecular formula decolorizes bromine water and forms a white precipitate in ethanolic solution Treatment of with in aqueous produces a compound, which gives a yellow precipitate when treated with and . The structure of is :
The major product of the following reaction is
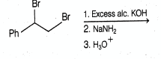
The structure of -methylpent--ene is
In the reaction of bromochlorocyclobutane with two equivalents of sodium in ether, the major product is
The reaction of an alkene with bromine produces a compound , Which has . The ozonolysis of alkene gives only one product. The alkene is [Given : atomic mass of ]
In the following reaction
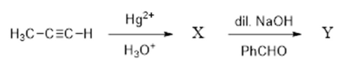
and , respectively, are
An organic compound X with molecular formula when treated with forms a gem dibromide. The compound X upon warming with and dil. produces a ketone, which gives a positive iodoform test. The compound X is
