Embibe Experts Solutions for Chapter: Thermodynamics, Exercise 1: Exercise 1
Embibe Experts Physics Solutions for Exercise - Embibe Experts Solutions for Chapter: Thermodynamics, Exercise 1: Exercise 1
Attempt the practice questions on Chapter 17: Thermodynamics, Exercise 1: Exercise 1 with hints and solutions to strengthen your understanding. Physics Crash Course COMEDK UGET solutions are prepared by Experienced Embibe Experts.
Questions from Embibe Experts Solutions for Chapter: Thermodynamics, Exercise 1: Exercise 1 with Hints & Solutions
If system is in thermal equilibrium with and is separately in thermal equilibrium with then and are in thermal equilibrium. From which thermodynamics law, does this follow?
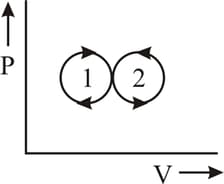
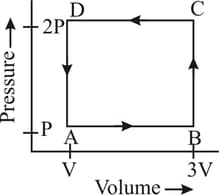
A thermodynamic system is taken through the cycle as shown in the figure. Heat rejected by the gas during the cycle is:
A gas mixture consists of 2 moles of oxygen and 4 moles of argon at temperature T. Neglecting all vibrational modes, the total internal energy of the system is:
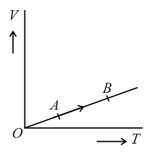
The volume of a monatomic gas varies with its temperature as shown in the graph. The ratio of work done by the gas to the heat absorbed by it, when it undergoes a change from state to state is?
and are specific heats at constant pressure and constant volume, respectively. It is observed that for hydrogen gas, for nitrogen gas. The correct relation between and is
Thermodynaic processes are indicated in the following diagram:
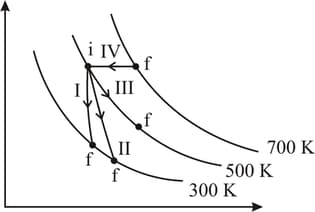
| Column-I | Column-II | ||
| P | Process I | a | Adiabatic |
| Q | Process II | b | Isobaric |
| R | Process III | c | Isochoric |
| S | Process IV | d | Isothermal |
A gas with the ratio of specific heat expands from an initial pressure of and volume to pressure and volume adiabatically. The work done is given by
The net amount of the work done in the following indicator diagram is -