Embibe Experts Solutions for Chapter: Thermodynamics, Exercise 1: JEE Main - 1 February 2023 Shift 1
Embibe Experts Physics Solutions for Exercise - Embibe Experts Solutions for Chapter: Thermodynamics, Exercise 1: JEE Main - 1 February 2023 Shift 1
Attempt the free practice questions on Chapter 10: Thermodynamics, Exercise 1: JEE Main - 1 February 2023 Shift 1 with hints and solutions to strengthen your understanding. EMBIBE CHAPTER WISE PREVIOUS YEAR PAPERS FOR PHYSICS solutions are prepared by Experienced Embibe Experts.
Questions from Embibe Experts Solutions for Chapter: Thermodynamics, Exercise 1: JEE Main - 1 February 2023 Shift 1 with Hints & Solutions
Given below are two statements : one is labelled as Assertion A and the other is labelled as Reason R.
Assertion A : Efficiency of a reversible heat engine will be highest at temperature of cold reservoir.
Reason R : The efficiency of Carnot’s engine depends not only on temperature of cold reservoir but it depends on the temperature of hot reservoir too and is given as
In the light of the above statements, choose the correct answer from the options given below :
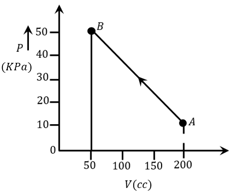
The pressure of a gas changes linearly with volume from to as shown in figure. If no heat is supplied to or extracted from the gas then change in the internal energy of the gas will be
The correct relation between and temperature is :
Heat energy of is given to a diatomic gas allowing the gas to expand at constant pressure. Each gas molecule rotates around an internal axis but do not oscillate. The increase in the internal energy of the gas will be:
A hypothetical gas expands adiabatically such that its volume changes from litres to litres. If the ratio of final pressure of the gas to initial pressure of the gas is . Then the ratio of will be.
A sample of gas at temperature is adiabatically expanded to double its volume. The work done by the gas in the process is given, (given ) :
A Carnot engine operating between two reservoirs has efficiency . When the temperature of cold reservoir raised by , its efficiency decreases to . The value of , if the temperature of hot reservoir is , will be
For three low density gases pressure versus temperature graphs are plotted while keeping them at constant volume, as shown in the figure
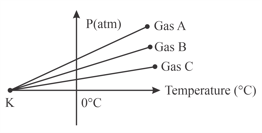
The temperature corresponding to the point is: