Embibe Experts Solutions for Chapter: Thermodynamics, Exercise 1: WBJEE 2020
Embibe Experts Physics Solutions for Exercise - Embibe Experts Solutions for Chapter: Thermodynamics, Exercise 1: WBJEE 2020
Attempt the free practice questions on Chapter 14: Thermodynamics, Exercise 1: WBJEE 2020 with hints and solutions to strengthen your understanding. EMBIBE CHAPTER WISE PREVIOUS YEAR PAPERS FOR PHYSICS solutions are prepared by Experienced Embibe Experts.
Questions from Embibe Experts Solutions for Chapter: Thermodynamics, Exercise 1: WBJEE 2020 with Hints & Solutions
Consider the given diagram. An ideal gas is contained in a chamber (left) of volume and is at an absolute temperature It is allowed to rush freely into the right chamber of volume which is initially vacuum. The whole system is thermally isolated. What will be the final temperature, if the equilibrium has been attained?
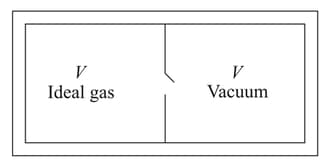
Which of the following statements(s) is/are true?
"Internal energy of an ideal gas.........
What will be the molar specific heat at constant volume of an ideal gas consisting of rigid diatomic molecules?
One mole of a monoatomic ideal gas undergoes a quasistatic process, which is depicted by a straight line joining points and in a diagram. What is the value of the heat capacity of the gas at the point
An ideal gas undergoes the cyclic process as shown in the given diagram.
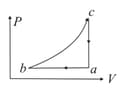
It rejects of heat during and absorbs of heat during . During , there is no transfer of heat and of work is done by the gas. What should be the area of the closed curve
The initial pressure and volume of a given mass of an ideal gas with , taken in a cylinder fitted with a piston, are and respectively. At this stage the gas has the same temperature as that of the surrounding medium which is It is adiabatically compressed to a volume equal to
Subsequently the gas is allowed to come to thermal equilibrium with the surroundings. What is the heat released to the surrounding?
For an ideal gas with initial pressure and volume and respectively, a reversible isothermal expansion happens, when its volume becomes . Then, it is compressed to its original volume by a reversible adiabatic process. If the final pressure is then which of the following statement(s) is/are true?
Consider an engine that absorbs of heat from a hot reservoir and delivers heat to a cold reservoir in each cycle. The engine also consumes energy in each cycle to overcome friction. If the engine works at cycles per minute, what will be the maximum power delivered to the load? [Assume the thermal equivalent of heat is ]
