Embibe Experts Solutions for Exercise 1: ICSE-2018
Embibe Experts Science Solutions for Exercise - Embibe Experts Solutions for Exercise 1: ICSE-2018
Attempt the free practice questions from Exercise 1: ICSE-2018 with hints and solutions to strengthen your understanding. EMBIBE CHAPTER WISE PREVIOUS YEAR PAPERS FOR SCIENCE solutions are prepared by Experienced Embibe Experts.
Questions from Embibe Experts Solutions for Exercise 1: ICSE-2018 with Hints & Solutions
State one relevant observation for of the following:
At the anode, when molten lead bromide is electrolyzed using graphite electrodes.
Name the gas that is produced in the following case:
At the anode during the electrolysis of acidified water.
For the electro-refining of copper, write the reaction that takes place at the anode.
Name the particles present in Weak electrolyte.
Identify the substance underlined in the following:
The electrode that increases in mass during the electro-refining of silver.
An aqueous solution of nickel (II) sulphate was electrolyzed using nickel electrodes. Observe the diagram and answer the question that follows:
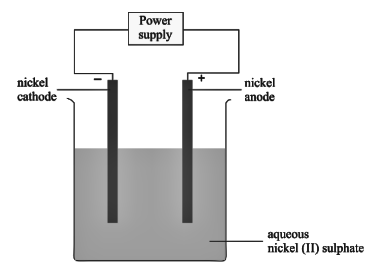
- What do you observe at the cathode and anode respectively?
An aqueous solution of nickel (II) sulphate was electrolyzed using nickel electrodes. Observe the diagram and answer the question that follows:
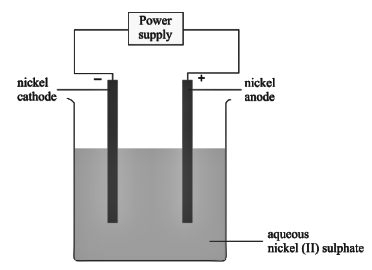
- Name the cation that remains as a spectator ion in the solution.
An aqueous solution of nickel (II) sulphate was electrolyzed using nickel electrodes. Observe the diagram and answer the question that follows:
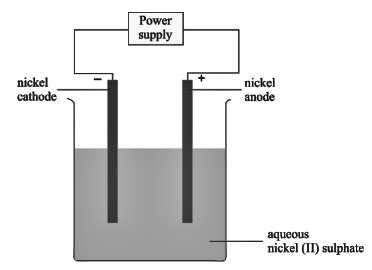
- Which equation for the reaction at the anode is correct?
