PRIYANKA B Solutions for Chapter: Metals and Non-Metals, Exercise 1: Value Based Questions
PRIYANKA B Chemistry Solutions for Exercise - PRIYANKA B Solutions for Chapter: Metals and Non-Metals, Exercise 1: Value Based Questions
Attempt the free practice questions on Chapter 3: Metals and Non-Metals, Exercise 1: Value Based Questions with hints and solutions to strengthen your understanding. ESSENTIALS OF CHEMISTRY X solutions are prepared by Experienced Embibe Experts.
Questions from PRIYANKA B Solutions for Chapter: Metals and Non-Metals, Exercise 1: Value Based Questions with Hints & Solutions
Raman took three metals labelled and . He carried out displacement reactions with his salt solutions and found that is less reactive than but more reactive than . The metals and respectively could be:
Rashmi added aluminum metal to colourless solution of zinc sulphate. After half an hour, the solution was observed. It was colourless.
She recorded her observations in the following statements:
(i) No reaction occurred
(ii) Reaction occurred and aluminium sulphate was formed
(iii) Zinc is more reactive than aluminium
(iv) Aluminium is more reactive than zinc.
The correct observations are:
Which of the following reactions will not proceed?
Ferrous sulphate crystals are dissolved in water. The colour of the solution would be
Mrignayani was doing the experiment of comparing the reactivity of metals in the laboratory. She was given aluminium metal and was told to check reactivity to by using four solutions as shown below. She would observe that reaction takes place in
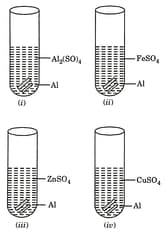
Which of the following statements are correct: Aluminium can displace
(i) from solution
(ii) from solution
(iii) from solution
(iv) from solution
Four test tubes marked and were taken. of solution in water was poured in each of the test tubes. A piece of zinc metal was placed in test tube , an iron nail was put in test tube , copper turnings were put in test tube and a clean aluminium strip was placed in test tube . No change was observed in any of the test tubes. The correct inference drawn is:
If iron nails are added in zinc sulphate solution, the possible observation could be:
