Embibe Experts Solutions for Chapter: Chemical Bonding and Molecular Structure, Exercise 3: Exercise-3
Author:Embibe Experts
Embibe Experts Chemistry Solutions for Exercise - Embibe Experts Solutions for Chapter: Chemical Bonding and Molecular Structure, Exercise 3: Exercise-3
Attempt the free practice questions on Chapter 14: Chemical Bonding and Molecular Structure, Exercise 3: Exercise-3 with hints and solutions to strengthen your understanding. Alpha Question Bank for Engineering: Chemistry solutions are prepared by Experienced Embibe Experts.
Questions from Embibe Experts Solutions for Chapter: Chemical Bonding and Molecular Structure, Exercise 3: Exercise-3 with Hints & Solutions
EASY
JEE Main/Advance
IMPORTANT
Molecular shapes of and are respectively:
EASY
JEE Main/Advance
IMPORTANT
The number of lone pair(s) of electrons in are:
HARD
JEE Main/Advance
IMPORTANT
The compound (s) with two lone pairs of electrons on the central atom is (are)
EASY
JEE Main/Advance
IMPORTANT
The number of lone pairs on in and respectively are:
EASY
JEE Main/Advance
IMPORTANT
Which of the following has the maximum number of lone pairs associated with
EASY
JEE Main/Advance
IMPORTANT
The correct statement for the molecule is:
EASY
JEE Main/Advance
IMPORTANT
For which of the following molecule significant
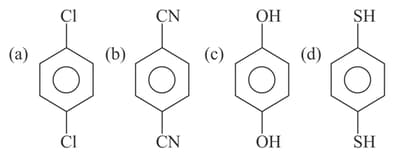
EASY
JEE Main/Advance
IMPORTANT
Among the following, the molecule with highest dipole moment is ?
