Exercise-3
Embibe Experts Chemistry Solutions for Exercise-3
Simple step-by-step solutions to Exercise-3 questions of Chemical Kinetics from Alpha Question Bank for Engineering: Chemistry. Also get 3D topic explainers, cheat sheets, and unlimited doubts solving on EMBIBE.
Questions from Exercise-3 with Hints & Solutions
The rate constant for the reaction is If the rate is then the concentration of (in ) is:
In the biologically -catalysed oxidation of ethanol, the concentration of ethanol decreases in a first order reaction from to in The constant rate of the reaction is:
Plots showing the variation of the rate constant with temperature are given below. The plot that follows Arrhenius equation is:
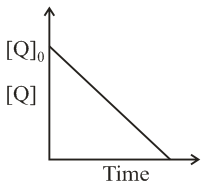
In the reaction,
the time taken for reaction of , is twice the time taken for reaction of The concentration of varies with reaction time as shown in the figure. The overall order of the reaction is:
For the reaction, the differential rate law can be written as:
The reaction of ozone with oxygen atoms in the presence of chlorine atoms can occur by a two-step process shown below:
The closest rate constant for the overall reaction is:
For an elementary chemical reaction,
the expressions for
The half life of a radio isotope is four hours. If the initial mass of the isotope was 200 g, the mass remaining after 24 hours undecayed is
