Exercise-2
Embibe Experts Chemistry Solutions for Exercise-2
Simple step-by-step solutions to Exercise-2 questions of Solutions from Alpha Question Bank for Engineering: Chemistry. Also get 3D topic explainers, cheat sheets, and unlimited doubts solving on EMBIBE.
Questions from Exercise-2 with Hints & Solutions
When of sulphuric acid solution is allowed to react with of sodium hydroxide solution, the amount of sodium sulphate (anhydrous) that can be obtained from the solution formed and the concentration of in the solution respectively are:
A sample of air is saturated with benzene(vapour pressure at ) at pressure. If it is isothermally compressed to one third of its initial volume, the final pressure of the system is
If vapour pressures of pure liquids '' and '' are and respectively at . When these two liquids are mixed at this temperature to form a solution in which mole percentage of '' is , then the total vapour pressure is observed to be . Which of the following is true for this solution?
on reaction with in aq. solution gives blue colour. These are separated by a semipermeable membrane as shown. Due to osmosis there is-
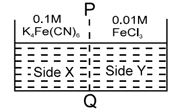
Select correct statements :
Two liquids and are perfectly immiscible. If and have molecular masses in ratio the total vapour pressure of a mixture of and prepared in weight ratio should be , ):
In the depression of freezing point experiment, it is found that the
What volume (in ) of alcohol by weight must be used to prepare of alcohol by weight ?
