Embibe Experts Solutions for Chapter: Solutions, Exercise 4: Exercise-4
Embibe Experts Chemistry Solutions for Exercise - Embibe Experts Solutions for Chapter: Solutions, Exercise 4: Exercise-4
Attempt the free practice questions on Chapter 8: Solutions, Exercise 4: Exercise-4 with hints and solutions to strengthen your understanding. Alpha Question Bank for Engineering: Chemistry solutions are prepared by Experienced Embibe Experts.
Questions from Embibe Experts Solutions for Chapter: Solutions, Exercise 4: Exercise-4 with Hints & Solutions
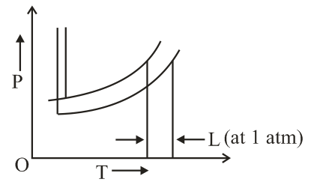
The phase diagrams for the pure solvent (solid lines) and the solution (non-volatile solute, dashed line) are recorded below: The quantity indicated by in the figure is:
Three different ideal solutions (I, II, III) each containing total moles of in different composition are taken as shown in figure and pressure over the solutions is gradually reduced.
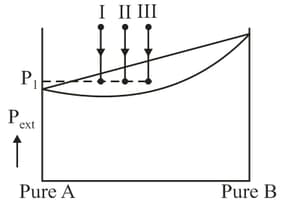
Initially external pressure is same for all three solutions. At a particular external pressure , ll solution is found to have
Then select correct statement:
For Henry's constant, which are correct?
The vapour pressure of ideal solution of benzene and toluene is at then what would be correct statement about same solution at
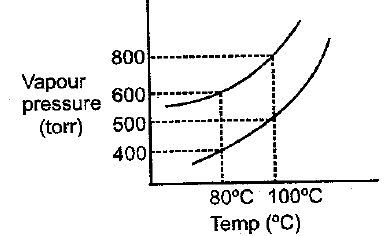
Select incorrect statement:
moles of and moles of are taken in separate beakers and enclosed in chamber Another moles of and moles of are mixed in a beaker and enclosed in chamber At equilibrium which of the following are not true. ( and are volatile liquids and they form ideal solution on mixing)
At the osmotic pressure of urea solution is The solution is diluted and the temperature is raised to when the osmotic pressure is found to be Determine extent of dilution.
A solution containing of a non volatile organic substance (Molecular mass ) in of benzene raises the boiling point of benzene by while a solution containing of another non-volatile substance in same amount of benzene raises the boiling point of benzene by If ratio of molecular masses of and is then. Find minimum value of
