Embibe Experts Solutions for Chapter: Dual Nature of Matter and Radiation, Exercise 3: Exercise-3
Embibe Experts Physics Solutions for Exercise - Embibe Experts Solutions for Chapter: Dual Nature of Matter and Radiation, Exercise 3: Exercise-3
Attempt the practice questions on Chapter 32: Dual Nature of Matter and Radiation, Exercise 3: Exercise-3 with hints and solutions to strengthen your understanding. Alpha Question Bank for Medical: Physics solutions are prepared by Experienced Embibe Experts.
Questions from Embibe Experts Solutions for Chapter: Dual Nature of Matter and Radiation, Exercise 3: Exercise-3 with Hints & Solutions
The number of photoelectrons emitted for light of a frequency is proportional to (higher than the threshold frequency )
Consider a situation When the monochromatic radiation of the intensity Falls on the metal surface, the number of photoelectrons and their maximum kinetic energy are and Respectively. If the intensity of radiation is then The number of emitted electrons and their maximum kinetic energy will be respectively
In the Davisson and Germer experiment, the velpcity of electrons emitted from the electron gun can be increased by:
An -particle moves in a circular path of radius in the presence of a magnetic field of The de Broglie wavelength associated with the particle will be
The wavelength of an electron and of a photon of same energy are related by
The photoelectric threshold wavelength of silver is . The velocity of the electron ejected from a silver surface by the ultraviolet light of wavelength is (Given, and )
An electron of mass with an initial velocity enters an electric field , (constant) at is its de Broglie wavelength at a time is
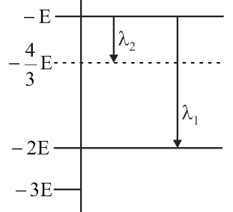
Some energy levels of a molecule are shown in the figure. The ratio of the wavelengths , is given by