Embibe Experts Solutions for Chapter: Chemical Kinetics, Exercise 2: EXERCISE-2
Embibe Experts Chemistry Solutions for Exercise - Embibe Experts Solutions for Chapter: Chemical Kinetics, Exercise 2: EXERCISE-2
Attempt the free practice questions on Chapter 10: Chemical Kinetics, Exercise 2: EXERCISE-2 with hints and solutions to strengthen your understanding. Beta Question Bank for Engineering: Chemistry solutions are prepared by Experienced Embibe Experts.
Questions from Embibe Experts Solutions for Chapter: Chemical Kinetics, Exercise 2: EXERCISE-2 with Hints & Solutions
At certain temperature, the half life period in the thermal decomposition of a gaseous substance as follows:
| (in min.) |
Find the order of reaction [Given
The rate of a reaction gets doubled when the temperature changes from to . By which factor will it change for the temperature change from to ?
For the reaction products (started with concentrations taken in stoichiometric proportion), the experimentally determined rate law is :
The half life time of the reaction would be:
A first order reaction is completed in minutes at . When same reaction in carried out at , it is complete in 5 minute. What is the activation energy of reaction (in )?
[Take : mole and
For the reaction , the rate law expression is . If initial concentration of is , then :
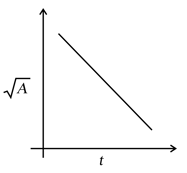
For the first order decomposition of ,
a graph of vs is shown in figure. What is the rate constant ?
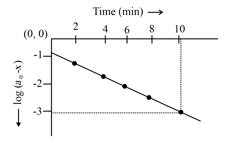
Consider the reaction , graph between half life and initial concentration (a) of the reactant is
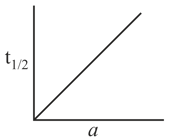
Hence, graph between And time will be
Select incorrect statement (s):
