Embibe Experts Solutions for Chapter: Organic Chemistry- Some Basic Principles and Techniques, Exercise 4: EXERCISE-4
Embibe Experts Chemistry Solutions for Exercise - Embibe Experts Solutions for Chapter: Organic Chemistry- Some Basic Principles and Techniques, Exercise 4: EXERCISE-4
Attempt the practice questions on Chapter 23: Organic Chemistry- Some Basic Principles and Techniques, Exercise 4: EXERCISE-4 with hints and solutions to strengthen your understanding. Beta Question Bank for Engineering: Chemistry solutions are prepared by Experienced Embibe Experts.
Questions from Embibe Experts Solutions for Chapter: Organic Chemistry- Some Basic Principles and Techniques, Exercise 4: EXERCISE-4 with Hints & Solutions
Benzene sulphonic acid is a stronger acid than benzoic acid, explain.
Which is a stronger acid, or and why ?
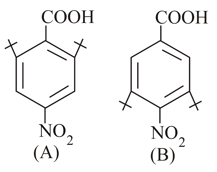
Which is a stronger acid, or and why ?
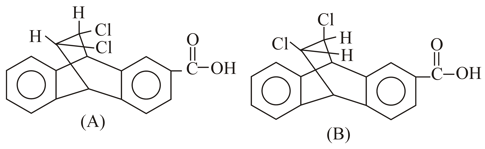
Rank the following sets of intermediates in increasing order of their stability giving appropriate reasons for your choice.
(a)
(b)
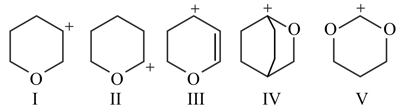
(c)
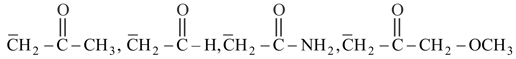
(acetate ion) is more stable than (ethoxide ion). Explain.
The sodium salt of a carboxylic acid, was produced by passing a gas, into an aqueous solution of caustic alkali at an elevated temperature and pressure. A on heating in presence of sodium hydroxide followed by treatment with sulphuric acid gave a dibasic acid A sameple of of on combustion gave of water and of carbondioxide. The silver salt of acid weighin on ignition yielded of silver as residue. Identify and
Discuss the following observations :
(c) is stronger acid than .
Discuss the following observations :
(d) is stronger base than .
