Embibe Experts Solutions for Chapter: Haloalkanes and Haloarenes, Exercise 3: EXERCISE-II (Previous Year Questions)
Embibe Experts Chemistry Solutions for Exercise - Embibe Experts Solutions for Chapter: Haloalkanes and Haloarenes, Exercise 3: EXERCISE-II (Previous Year Questions)
Attempt the practice questions on Chapter 23: Haloalkanes and Haloarenes, Exercise 3: EXERCISE-II (Previous Year Questions) with hints and solutions to strengthen your understanding. Beta Question Bank for Medical: Chemistry solutions are prepared by Experienced Embibe Experts.
Questions from Embibe Experts Solutions for Chapter: Haloalkanes and Haloarenes, Exercise 3: EXERCISE-II (Previous Year Questions) with Hints & Solutions
Isobutyl magnesium bromide with dry ether and absolute alcohol gives
Identify in the following reaction series,
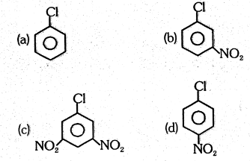
Arrange the following compound in increasing order of reactivity towards aromatic nucleophilic substitution reaction.
Chloroform when treated with benzene in presence of anhydrous the product formed is
, in the above reaction sequence is:
Major product of the reaction :- would be :-
Chlorobenzene and benzyl chloride can be distinguished by treatment with .
Chlorobenzene does not gives white ppt with due to resonance.
Benzyl phenylether forms phenol and benzyl iodide with
Because benzyl carbocations is more stable than phenyl.carbocation.
