Embibe Experts Solutions for Chapter: Thermodynamics, Exercise 4: EXERCISE-III (Analytical Questions)
Embibe Experts Chemistry Solutions for Exercise - Embibe Experts Solutions for Chapter: Thermodynamics, Exercise 4: EXERCISE-III (Analytical Questions)
Attempt the practice questions on Chapter 4: Thermodynamics, Exercise 4: EXERCISE-III (Analytical Questions) with hints and solutions to strengthen your understanding. Beta Question Bank for Medical: Chemistry solutions are prepared by Experienced Embibe Experts.
Questions from Embibe Experts Solutions for Chapter: Thermodynamics, Exercise 4: EXERCISE-III (Analytical Questions) with Hints & Solutions
For a reversible process at the volume is increased from to . Calculate if the process is isothermal:
The entropy change involved in the isothermal reversible expansion of of an ideal gas from a volume of to a volume of at is:
The conversion to is carried out by the following path :
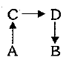
Given :
,
where is entropy unit then is
In conversion of lime-stone to lime, the values and are and respectively at and . Assuming that and do not change with temperature, temperature above which conversion of lime stone to lime will be spontaneous is :
Identify the correct statement regarding a sponateous process :-
For complete combustion of ethanol, , the amount of heat produced as measured in bomb calorimeter, is at . Assuming ideality the enthalpy of combustion, reaction will be :
If the bond dissociation energies of(all diatomic molecules) are in the ratio and for the formation of is , the bond dissociation energy of will be
Oxidising power of chlorine in an aqueous solution can be determined by the parameters indicated below:
The energy involved in the conversion of
to
(using the data, , ) will be:
