Embibe Experts Solutions for Chapter: The d- and f-Block Elements, Exercise 1: Exercise 1
Embibe Experts Chemistry Solutions for Exercise - Embibe Experts Solutions for Chapter: The d- and f-Block Elements, Exercise 1: Exercise 1
Attempt the practice questions on Chapter 21: The d- and f-Block Elements, Exercise 1: Exercise 1 with hints and solutions to strengthen your understanding. Chemistry Crash Course JEE Advanced solutions are prepared by Experienced Embibe Experts.
Questions from Embibe Experts Solutions for Chapter: The d- and f-Block Elements, Exercise 1: Exercise 1 with Hints & Solutions
Products
Reaction products are
(Ammonium dichromate) is used in fire works. The green coloured powder blown in air is:
 and Are respectively :
and Are respectively :
Increasing value of magnetic moment of following species is
. .
A black mineral on heating in the presence of air gives a pungent smelling gas. on reaction with dilute gives gas and a solution of compound. On passing gas into aqueous solution of , a white turbidity is obtained. The aqueous solution of on reaction with gives a blue precipitate. Compound and are respectively.
A black mineral in solid state is fused with and and the mixture extracted with water to get a green coloured solution . On passing gas through the solution the colour changes to pink with a black residue . Which of the following is/are correct
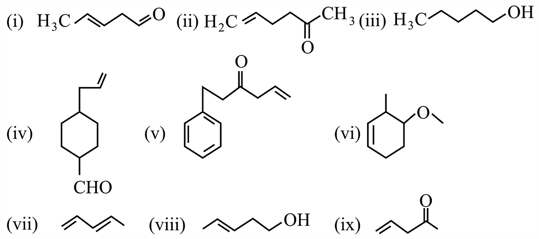
Identify the total number of compounds which does not make yellow precipitate with -DNP but show positive test with cold alkaline .
How many number of following ions for which all octahedral complexes are paramagnetic irrespective of ligand
