Embibe Experts Solutions for Chapter: Chemical Bonding and Molecular Structure, Exercise 2: Level 2
Embibe Experts Chemistry Solutions for Exercise - Embibe Experts Solutions for Chapter: Chemical Bonding and Molecular Structure, Exercise 2: Level 2
Attempt the practice questions on Chapter 13: Chemical Bonding and Molecular Structure, Exercise 2: Level 2 with hints and solutions to strengthen your understanding. Chemistry Crash Course NEET solutions are prepared by Experienced Embibe Experts.
Questions from Embibe Experts Solutions for Chapter: Chemical Bonding and Molecular Structure, Exercise 2: Level 2 with Hints & Solutions
The hybridisation of the carbon atom gives rise to the ____ structure.
Which of the following is an incorrect rule for drawing resonance structures?
Which of the following is planar?
Which will have higher dipole moment than 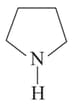 ?
?
The correct order for the given pair of isomers is:
Among the following transformations, the hybridization of the central atom remains unchanged in:
The correct order of boiling points of the following compounds is:
I 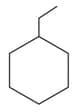
II 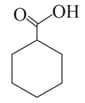
III 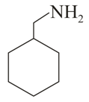
IV 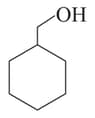
Among the following, the compound that has the highest dipole moment is:
