Embibe Experts Solutions for Chapter: Equilibrium, Exercise 3: Level 3
Embibe Experts Chemistry Solutions for Exercise - Embibe Experts Solutions for Chapter: Equilibrium, Exercise 3: Level 3
Attempt the practice questions on Chapter 5: Equilibrium, Exercise 3: Level 3 with hints and solutions to strengthen your understanding. Chemistry Crash Course NEET solutions are prepared by Experienced Embibe Experts.
Questions from Embibe Experts Solutions for Chapter: Equilibrium, Exercise 3: Level 3 with Hints & Solutions
Buffer capacity of a buffer solution is , the volume of added to of this solution if change the by is:
What fraction of an indicator is in basic form at a of if the of the indicator is
An acid-base indicator which is a weak acid has a value . At what concentration ratio of sodium acetate to acetic acid would the indicator show a colour halfway between those of its acid and conjugate base forms? [ of acetic acid ]
The solubility curve of as a function of temperature is given below:
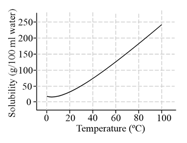
The amount of that will crystallize when a saturated solution of in of water is cooled from to , is:
The reaction,
is begun with the concentrations of and both at an initial value of When equilibrium is reached, the concentration of is measured and found to be The value for the equilibrium constant for this reaction is given by the expression:
At what will a solution of an indicator indicator change colour?
Calculate the of a solution for carbonic acid. The reactions are:
Blood plasma is maintained at a of largely by the
