Embibe Experts Solutions for Chapter: Chemical Kinetics, Exercise 1: BITSAT 2017
Embibe Experts Chemistry Solutions for Exercise - Embibe Experts Solutions for Chapter: Chemical Kinetics, Exercise 1: BITSAT 2017
Attempt the free practice questions on Chapter 11: Chemical Kinetics, Exercise 1: BITSAT 2017 with hints and solutions to strengthen your understanding. EMBIBE CHAPTER WISE PREVIOUS YEAR PAPERS FOR CHEMISTRY solutions are prepared by Experienced Embibe Experts.
Questions from Embibe Experts Solutions for Chapter: Chemical Kinetics, Exercise 1: BITSAT 2017 with Hints & Solutions
Given the hypothetical reaction mechanism
and the rate as
| Species formed | Rate of its information |
| per mole of | |
| per mole of | |
| per mole of | |
| per mole of |
The rate determining step is
For the chemical reaction
, the reaction proceeds as follows
(Fast)
, (Slow)
the rate law expression should be given as
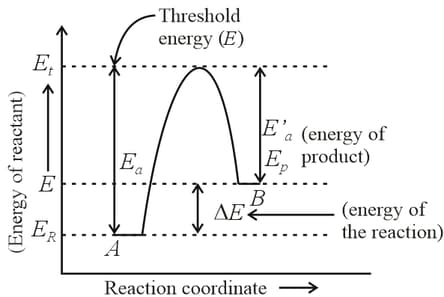
For a reversible reaction, , which one of the following statement is wrong from the given energy profile diagram?
Under the same reaction conditions, initial concentration of of a substance becomes half in and through first order and zero order kinetics, respectively. Ratio of the rate of constants for first order and zero order of the reaction is
The rate of a reaction triples when temperature changes from to . The energy of activation for the reaction is
Which of the following expression is correct for the rate of reaction given below ?
For an endothermic reaction, where represent the enthalpy of reaction in , the minimum value for energy of activation (for forward reaction) will be
A graph is plotted between log versus for calculation of activation energy . The correct plot is
