Embibe Experts Solutions for Chapter: Chemical Kinetics, Exercise 1: JEE Advanced Paper 1 - 2020
Embibe Experts Chemistry Solutions for Exercise - Embibe Experts Solutions for Chapter: Chemical Kinetics, Exercise 1: JEE Advanced Paper 1 - 2020
Attempt the free practice questions on Chapter 10: Chemical Kinetics, Exercise 1: JEE Advanced Paper 1 - 2020 with hints and solutions to strengthen your understanding. EMBIBE CHAPTER WISE PREVIOUS YEAR PAPERS FOR CHEMISTRY solutions are prepared by Experienced Embibe Experts.
Questions from Embibe Experts Solutions for Chapter: Chemical Kinetics, Exercise 1: JEE Advanced Paper 1 - 2020 with Hints & Solutions
For the following reaction the rate of reaction is . Two moles of are mixed with one mole of to make of solution. At mole of is left in the reaction mixture. The correct statement(s) about the reaction is(are) Use:
Which of the following plots is(are) correct for the given reaction?
( is the initial concentration of )
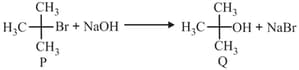
The decomposition reaction is started in a closed cylinder under the isothermal isochoric condition at an initial pressure of . After , the pressure inside the cylinder is found to be . If the rate constant of the reaction is , assuming ideal gas behaviour, the value of is(nearest integer)
For a first order reaction at constant volume and 300 K, the total pressure at the beginning and at time are , respectively. Initially, only A is present with concentration , and is the time required for the partial pressure of A to reach of its initial value. The correct option(s) is (are)
(Assume that all these gases behave as ideal gases)
Consider the following reversible reaction:
The activation energy of the backward reaction exceeds that of the forward reaction by (in ). If the pre-exponential factor of the forward reaction is times that of the reverse reaction, the absolute value of for the reaction at is ____.
(Given; and is the Gibbs energy)
In a bimolecular reaction, the steric factor was experimentally determined to be . The correct option(s) among the following is(are)
is known to undergo radioactive decay to form by emitting alpha and beta particles. A rock initially contained of . If the number of alpha particles that it would emit during its radioactive decay of to in three half-lives is , then what is the value of ?
Consider the kinetic data given in the following table for the reaction Product.
| Experiment No. | Rate of reaction | |||
The rate of the reaction for and is found to be The value of is __________
