Embibe Experts Solutions for Chapter: Organic Chemistry- Some Basic Principles and Techniques, Exercise 1: JEE Main - 1 February 2023 Shift 1
Embibe Experts Chemistry Solutions for Exercise - Embibe Experts Solutions for Chapter: Organic Chemistry- Some Basic Principles and Techniques, Exercise 1: JEE Main - 1 February 2023 Shift 1
Attempt the free practice questions on Chapter 22: Organic Chemistry- Some Basic Principles and Techniques, Exercise 1: JEE Main - 1 February 2023 Shift 1 with hints and solutions to strengthen your understanding. EMBIBE CHAPTER WISE PREVIOUS YEAR PAPERS FOR CHEMISTRY solutions are prepared by Experienced Embibe Experts.
Questions from Embibe Experts Solutions for Chapter: Organic Chemistry- Some Basic Principles and Techniques, Exercise 1: JEE Main - 1 February 2023 Shift 1 with Hints & Solutions
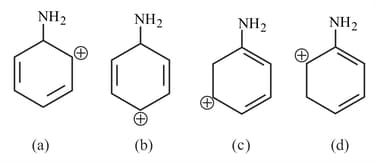
The most stable carbocation for the following is:
Match items of column I and II
| Column I (Mixture of compounds) | Column II (Separation Technique) |
| A. | i. Crystallization |
|
B.
|
ii. Differential solvent extraction |
| C. Kerosene/Naphthalene | iii. Column chromatography |
| D. | iv. Fractional Distillation |
In Dumas method for the estimation of , the sample is heated with copper oxide and the gas evolved is passed over:
When a hydrocarbon undergoes complete combustion it requires equivalents of oxygen and produces equivalents of water. What is the molecular formula of ?
A solution of when treated with gives a prussian blue precipitate due to the formation of
Number of isomeric compounds with molecular formula which
(i) do not dissolve in
(ii) do not dissolve in .
(iii) do not give orange precipitate with - DNP
(iv) on hydrogenation give identical compound with molecular formula is
All structures given below are of vitamin . Most stable of them is:
Given below are two statements:
Statement I: Sulphanilic acid gives esterification test for carboxyl group.
Statement II: Sulphanilic acid gives red colour in Lassigne’s test for extra element detection.
In the light of the above statements, choose the most appropriate answer from the options given below: