Embibe Experts Solutions for Chapter: States of Matter : Gases and Liquids, Exercise 1: AP EAPCET - 20 Aug 2021 Afternoon
Embibe Experts Chemistry Solutions for Exercise - Embibe Experts Solutions for Chapter: States of Matter : Gases and Liquids, Exercise 1: AP EAPCET - 20 Aug 2021 Afternoon
Attempt the free practice questions on Chapter 9: States of Matter : Gases and Liquids, Exercise 1: AP EAPCET - 20 Aug 2021 Afternoon with hints and solutions to strengthen your understanding. EMBIBE CHAPTER WISE PREVIOUS YEAR PAPERS FOR CHEMISTRY solutions are prepared by Experienced Embibe Experts.
Questions from Embibe Experts Solutions for Chapter: States of Matter : Gases and Liquids, Exercise 1: AP EAPCET - 20 Aug 2021 Afternoon with Hints & Solutions
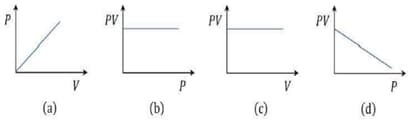
Which of the following graphs correctly represents Boyle's Law?
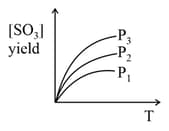
For the reaction , the percentage yield of product at different pressures is shown in the figure. Then, which among the following is true?
Three flasks of equal volumes contain gases respectively. They will contain equal number of molecules if
Which among the following statements is/are incorrect regarding real gases?
(i) Their compressibility factor is never equal to unity .
(ii) The deviations from ideal behavior are less at low pressures and high temperatures.
(iii) Intermolecular forces among gas molecules are equal to zero.
(iv) The obey Van der Waals equation, .
The density of an ideal gas can be given by where and respectively denote pressure, volume, molar-mass, temperature and universal gas constant.
Among the following, the maximum deviation from ideal gas behavior is expected from
Two flasks and have equal volumes. is maintained at and at . Equal masses of and are taken in flasks and respectively. Find the ratio of total of gases in flask to that of .
When the temperature of a gas is increased from to , the root mean square speed of the gas would
