Embibe Experts Solutions for Chapter: States of Matter : Gases and Liquids, Exercise 1: AP EAPCET Medical 2019 (24-Apr Shift-1)
Embibe Experts Chemistry Solutions for Exercise - Embibe Experts Solutions for Chapter: States of Matter : Gases and Liquids, Exercise 1: AP EAPCET Medical 2019 (24-Apr Shift-1)
Attempt the practice questions on Chapter 5: States of Matter : Gases and Liquids, Exercise 1: AP EAPCET Medical 2019 (24-Apr Shift-1) with hints and solutions to strengthen your understanding. EMBIBE CHAPTER WISE PREVIOUS YEAR PAPERS FOR CHEMISTRY solutions are prepared by Experienced Embibe Experts.
Questions from Embibe Experts Solutions for Chapter: States of Matter : Gases and Liquids, Exercise 1: AP EAPCET Medical 2019 (24-Apr Shift-1) with Hints & Solutions
At constant temperature, when a bulb of volume containing an ideal gas was connected to another evacuated bulb the pressure fell down by of bulb A's initial pressure. Then, find the volume of bulb
Calculate the volume occupied by of water vapour at and bar pressure.
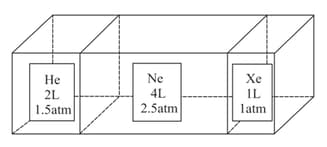
Consider the composite system, which is held at shown in the figure. Assuming ideal gas behavior, calculate the total pressure if the barriers separating the compartments are removed. Assume that the volume of the barriers is negligible.
Calculate the density of gas at and pressure.
An evacuated glass vessel weighs when empty, when filled with a liquid of density and when filled with an ideal gas at at Then find the molar mass of the ideal gas.
At what temperature in, the most probable velocity of ozone gas is equal to RMS velocity of oxygen gas at
The order of kinetic diameter of and is:
Given the critical temperature and critical pressure of gas are and respectively, find the approximate values of Van der Waals constants (in atm. ) and (in L. ).
