Embibe Experts Solutions for Chapter: Thermal Properties of Matter, Exercise 1: JEE Main - 26 June 2022 Shift 2
Embibe Experts Physics Solutions for Exercise - Embibe Experts Solutions for Chapter: Thermal Properties of Matter, Exercise 1: JEE Main - 26 June 2022 Shift 2
Attempt the free practice questions on Chapter 8: Thermal Properties of Matter, Exercise 1: JEE Main - 26 June 2022 Shift 2 with hints and solutions to strengthen your understanding. EMBIBE CHAPTER WISE PREVIOUS YEAR PAPERS FOR PHYSICS solutions are prepared by Experienced Embibe Experts.
Questions from Embibe Experts Solutions for Chapter: Thermal Properties of Matter, Exercise 1: JEE Main - 26 June 2022 Shift 2 with Hints & Solutions
Two metallic wires of identical dimensions are connected is series. If and are the conductivities of the these wires respectively, the effective conductivity of the combination is :
A hole is drilled in a metal sheet. At , the diameter of hole is . When the sheet is heated to , the change in the diameter of hole is . The value of will be _____, if coefficient of linear expansion of the metal is
A bowl filled with very hot soup cools from to $86^{\circ} \mathrm{C}$ in 2 minutes when the room temperature is $22^{\circ} \mathrm{C}$. How long it will take to cool from $75^{\circ} \mathrm{C}$ to $69^{\circ} \mathrm{C}$ ?
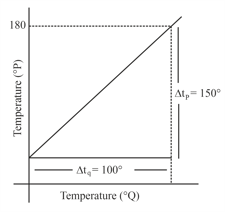
The graph between two temperature scales and is shown in the figure. Between upper fixed point and lower fixed point there are equal divisions of scale and divisions on scale . The relationship for conversion between the two scales is given by :
 .
.
A body cools from to in minutes. If, temperature of surroundings is . Then, after the next minutes, its temperature will be ______ .
Heat energy of is given to ice of mass at , Specific heat of ice is , and the latent heat of ice is . Consider the statements below.
(A) Final temperature of the system will be
(B) Final temperature of the system will be greater than
(C) The final system will have a mixture of ice and water in the ratio of
(D) The final system will have a mixture of ice and water in the ratio of
(E) The final system will have water only
Choose the correct answer from the options given below :
The pressure and temperature relationship of an ideal gas obeys the equation constant. The volume expansion coefficient of the gas will be :
A faulty thermometer reads in melting ice and in steam. The correct temperature on absolute scale will be ______ when the faulty thermometer reads .
