Embibe Experts Solutions for Chapter: Thermodynamics, Exercise 1: TS EAMCET - 5 August 2021 Shift 1
Embibe Experts Physics Solutions for Exercise - Embibe Experts Solutions for Chapter: Thermodynamics, Exercise 1: TS EAMCET - 5 August 2021 Shift 1
Attempt the free practice questions on Chapter 17: Thermodynamics, Exercise 1: TS EAMCET - 5 August 2021 Shift 1 with hints and solutions to strengthen your understanding. EMBIBE CHAPTER WISE PREVIOUS YEAR PAPERS FOR PHYSICS solutions are prepared by Experienced Embibe Experts.
Questions from Embibe Experts Solutions for Chapter: Thermodynamics, Exercise 1: TS EAMCET - 5 August 2021 Shift 1 with Hints & Solutions
A diatomic gas of volume at pressure is compressed adiabatically to a volume The work done in this process is,
[Use ]
of water is heated from to What is the change in its internal energy? (Specific heat of water is )
A tyre pumped to a pressure of atmosphere, suddenly bursts. If the temperature of air before expansion is then the air temperature after the tyre busts is
(Assume the expansion is adiabatic and adiabatic constant
An ideal gas undergoes an adiabatic process. If the pressure of the gas is reduced by then the volume is changed by (Given )
An ideal monoatomic gas of volume is adiabatically expanded to a volume at The final temperature in Kelvins is
(use )
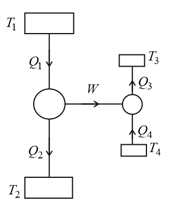
Consider a two stage Carnot engine. In the first stage heat is absorbed at temperature and heat is expelled at temperature (where ). In the second stage heat is absorbed at temperature and heat is expelled at temperature The efficiency of the Carnot engine will be
The carnot heat engine have an efficiency of The temperature of sink is maintained at To increase the efficiency upto the increment in the source temperature is
The following figure shows a Carnot engine that works between temperatures and and drives a Carnot refrigeration that works between temperatures and The quantity will be