Embibe Experts Solutions for Chapter: Chemical Reactions and Equations, Exercise 1: Jharkhand Board-2018
Embibe Experts Science Solutions for Exercise - Embibe Experts Solutions for Chapter: Chemical Reactions and Equations, Exercise 1: Jharkhand Board-2018
Attempt the practice questions on Chapter 1: Chemical Reactions and Equations, Exercise 1: Jharkhand Board-2018 with hints and solutions to strengthen your understanding. EMBIBE CHAPTER WISE PREVIOUS YEAR PAPERS FOR SCIENCE solutions are prepared by Experienced Embibe Experts.
Questions from Embibe Experts Solutions for Chapter: Chemical Reactions and Equations, Exercise 1: Jharkhand Board-2018 with Hints & Solutions
is which type of reaction?
What do you mean by a precipitation reaction?
Explain oxidation and reduction in terms of gain or loss of oxygen. Mention examples for each one.
Balance the following chemical equations:
(a)
(b)
Explain oxidation and reduction in terms of gain or loss of oxygen. Mention one example for each one.
The chemical reaction is which type of reaction?
Balance the following chemical equations:
(a)
(b)
The reaction of water with has been shown in the given diagram.
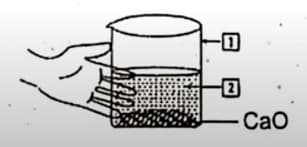
(a) Write the names of and .
(b) Write the name of the reaction taking place.
(c) Write the balanced equation for the reaction taking place.
