Embibe Experts Solutions for Exercise 5: Assignment
Embibe Experts Physics Solutions for Exercise - Embibe Experts Solutions for Exercise 5: Assignment
Attempt the free practice questions from Exercise 5: Assignment with hints and solutions to strengthen your understanding. Gamma Question Bank for Engineering Physics solutions are prepared by Experienced Embibe Experts.
Questions from Embibe Experts Solutions for Exercise 5: Assignment with Hints & Solutions
Two thermally insulated vessels and are filled with air at temperatures volume and pressure respectively. If the valve joining the two vessels is opened, the temperature inside the vessel at equilibrium will be
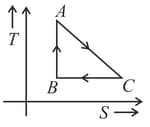
As area under diagram represent work done by gas in a thermodynamic process, area under temperature entropy graph represents heat supplied to the thermodynamic system. Consider the following graph
One mole of argon is expanded according to process equation constant and its temperature falls by then
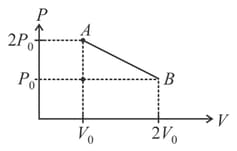
Two moles of an ideal monatomic gas is taken through cycle as shown. Then select the correct statement The symbols have their usual meaning
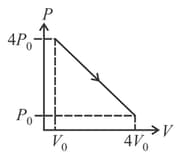
If the indicator diagram for expansion of gas is as shown, the gas
When a certain amount of heat is supplied to a diatomic gas at constant volume, rise in temperature is When same heat is supplied to the gas at constant pressure, what is the rise in temperature?
A cylinder of length has a thin massless piston fixed in such a way that it divides the cylinder into two equal parts. The cylinder is kept in a large heat bath at temperature The left side of the piston contains moles of an ideal gas at pressure and the right side contains the same gas at pressure Now the piston is released. The heat absorbed by the gas in the process of equilibration is being integers. The value of is
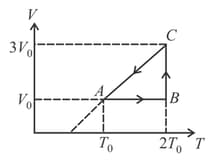
In the given diagram, if temperature at is then maximum temperature during the process is_____