Embibe Experts Solutions for Exercise 3: Assignment
Embibe Experts Chemistry Solutions for Exercise - Embibe Experts Solutions for Exercise 3: Assignment
Attempt the free practice questions from Exercise 3: Assignment with hints and solutions to strengthen your understanding. Gamma Question Bank for Medical Chemistry solutions are prepared by Experienced Embibe Experts.
Questions from Embibe Experts Solutions for Exercise 3: Assignment with Hints & Solutions
The reaction follows first order kinetics. The time taken for mole of A to produce mole of is 1 hour. What is the time taken for conversion of mole of to produce mole of ?
If
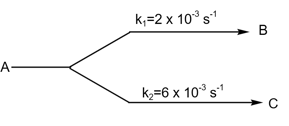
find
The graph between the versus is a straight line. The slope of the line is
The temperature coefficient for most of the reaction lies between
Which of the following is correct?
The rate constant of a reaction is at and at . What is the value of activation energy?
In Arrhenius equation, may not be termed as rate constant
If the rate of reaction increases by 27 times, when temperature is increased by , then temperature coefficient of the reaction is
