Embibe Experts Solutions for Exercise 1: EXERCISE
Embibe Experts Chemistry Solutions for Exercise - Embibe Experts Solutions for Exercise 1: EXERCISE
Attempt the practice questions from Exercise 1: EXERCISE with hints and solutions to strengthen your understanding. Gamma Question Bank for Medical Chemistry solutions are prepared by Experienced Embibe Experts.
Questions from Embibe Experts Solutions for Exercise 1: EXERCISE with Hints & Solutions
If the rate of reaction increases to two times with every rise in temperature, then if we raise the temperature by , the new rate of the reaction will be
According to collision theory, rate of the reaction increases, because
is . What is the activation energy for the backward reaction?
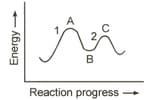
Rate of reaction depends upon
The correct expression for Arrhenius equation is (where is rate constant)
The temperature coefficient of a certain reaction is found to be . If temperature changes from to , the new rate of reaction will be
In Arrhenius equation, . The is
