Embibe Experts Solutions for Exercise 2: Assignment
Embibe Experts Chemistry Solutions for Exercise - Embibe Experts Solutions for Exercise 2: Assignment
Attempt the free practice questions from Exercise 2: Assignment with hints and solutions to strengthen your understanding. Gamma Question Bank for Medical Chemistry solutions are prepared by Experienced Embibe Experts.
Questions from Embibe Experts Solutions for Exercise 2: Assignment with Hints & Solutions
Which of the following reactions has minimum value of
Consider the following graph for the chemical reaction
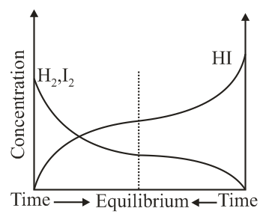
The inference that can be obtained from this graph about chemical equilibrium is that
Out of the following, choose an incorrect statement.
The and value of acetic acid and ammonium hydroxide are and respectively, then the of ammonium acetate solution is
The dissociation constant of a weak monoacidic base is The of its solution will be approximately equal to
For the above two reactions which of the given relation is correct?
What is the of at
The solubility of sparingly soluble salt is Its solubility product is
