Embibe Experts Solutions for Exercise 3: Assignment
Embibe Experts Chemistry Solutions for Exercise - Embibe Experts Solutions for Exercise 3: Assignment
Attempt the practice questions from Exercise 3: Assignment with hints and solutions to strengthen your understanding. Gamma Question Bank for Medical Chemistry solutions are prepared by Experienced Embibe Experts.
Questions from Embibe Experts Solutions for Exercise 3: Assignment with Hints & Solutions
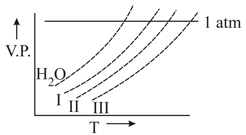
Which is having highet elevation in boiling point?
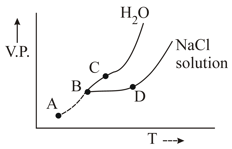
Freezing point of solution is marked as
Van't Hoff factor for acetic acid in aqueous medium at infinite dilution is
Correct oder of freezing point of given solution
I. glucose
II. urea
III.
IV.
Boiling point of which is dissociated in aqueous medium as and
If any solute dimerises in water at pressure and the boiling point of this solution is . If moles of is added to of water and for water is, calculate the percentage association of
Substance tetramerises in water to the extent of . A solution of of in of water lowers the freezing point by . The molar mass of is for water
is supposed to be dissociated when solution prepared. Its boiling point is equal to another mass by volume of non-electrolytic solution A. Considering molality = molarity. The molecular weight of is
