Embibe Experts Solutions for Chapter: Thermodynamics, Exercise 1: Exercise 1
Embibe Experts Physics Solutions for Exercise - Embibe Experts Solutions for Chapter: Thermodynamics, Exercise 1: Exercise 1
Attempt the practice questions on Chapter 12: Thermodynamics, Exercise 1: Exercise 1 with hints and solutions to strengthen your understanding. Physics Crash Course JEE Advanced solutions are prepared by Experienced Embibe Experts.
Questions from Embibe Experts Solutions for Chapter: Thermodynamics, Exercise 1: Exercise 1 with Hints & Solutions
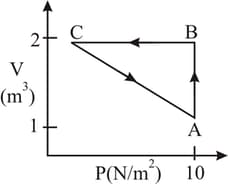
An ideal gas is taken through the cycle as shown in the fig. If the net heat supplied to the gas in the cycle is the work done by the gas in the process is:
Consider to be the universal gas constant. Given that, the amount of heat needed to raise the temperature of of an ideal monatomic gas from to when no work is done is . Then is
of is contained in a cubical container of side and maintained at a temperature of . The isothermal bulk modulus of elasticity of the gas in terms of universal gas constant is . Find the value of .
An ideal gas is taken through a process in which the pressure and the volume vary such that . The value of , for which molar heat capacity of gas is zero, is . Value of , is
An ideal monoatomic gas undergoes a process such that its internal energy relates to the volume as , where is a constant. Molar specific heat of the gas is Value of is
Two moles of a diatomic ideal gas is taken through a process, , where is a positive constant. Temperature of gas is increased from to , work done by the gas is . Value of , is
A cyclic process is performed on one mole of an ideal gas, and . A total of of heat is withdrawn from the gas in the complete cycle. Work done by the gas during process is . Value of is
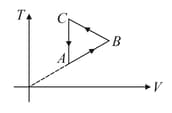
moles of a diatomic gas performs a work on its surrounding when amount of heat is supplied to the gas. Molar heat capacity of the process is . Value of , is
