Embibe Experts Solutions for Chapter: Dual Nature of Matter and Radiation, Exercise 1: Level 1
Embibe Experts Physics Solutions for Exercise - Embibe Experts Solutions for Chapter: Dual Nature of Matter and Radiation, Exercise 1: Level 1
Attempt the practice questions on Chapter 20: Dual Nature of Matter and Radiation, Exercise 1: Level 1 with hints and solutions to strengthen your understanding. Physics Crash Course JEE Main solutions are prepared by Experienced Embibe Experts.
Questions from Embibe Experts Solutions for Chapter: Dual Nature of Matter and Radiation, Exercise 1: Level 1 with Hints & Solutions
The ratio of the de Broglie wavelength of a particle to that of an electron is . The mass of the particle, if it moves three times faster than the electron is . Then the value of is: (Approximate the answer to nearest integer)
A particle of mass is moving with a velocity . Its de-Broglie wavelength is nearly
If the momentum of electron is changed by then the de Broglie wavelength associated with it changes by The initial momentum of electron will be:
The wavelength of an electron and of a photon of same energy are related by:
A photon of wavelength is absorbed by an electron confined to a box of length . As a result, the electron makes a transition from state, to the state . Subsequently, the electron transits from the state to the state by emitting a photon of wavelength, . Then,
Photon of frequency has a momentum associated with it. If is the velocity of light, the momentum is
A photosensitive surface is irradiated with light of wavelength , the stopping potential is . When the same surface is irradiated with the light of wavelength , stopping potential is . The ratio of threshold wavelength and the is . Write the value of .
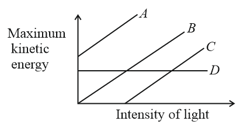
The graph between maximum kinetic energy and intensity of light in photoelectric effect is plotted. Out of the four graphs shown in the figure, the correct graph is