Embibe Experts Solutions for Chapter: Thermodynamics, Exercise 3: Level 3
Embibe Experts Physics Solutions for Exercise - Embibe Experts Solutions for Chapter: Thermodynamics, Exercise 3: Level 3
Attempt the practice questions on Chapter 10: Thermodynamics, Exercise 3: Level 3 with hints and solutions to strengthen your understanding. Physics Crash Course JEE Main solutions are prepared by Experienced Embibe Experts.
Questions from Embibe Experts Solutions for Chapter: Thermodynamics, Exercise 3: Level 3 with Hints & Solutions
One mole of a monoatomic gas and one mole of a diatomic gas are initially in the same state. Both gases are expanded isothermally and then adiabatically, such that they acquire the same final state. Choose the correct statement.
An ideal gas is made to undergo the cyclic process shown in the figure below.
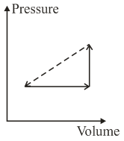
Let depict the work done, be the change in internal energy of the gas and be the heat added to the gas. Sign of each of these three quantities for the whole cycle will be ( refers to no change)
In a cyclic process for an ideal gas. In and process and heat is supplied to an ideal gas. In process internal energy of gas increases by and in process work done by gas is . The increase in internal energy of gas in process is
For an ideal gas, the internal energy is given by where is a constant. The equation of the adiabats in the -plane will be
A thermally insulated vessel contains an ideal gas of molecular mass and a ratio of specific heats . It is moving with speed and is suddenly brought to rest. Assuming no heat is lost to the surroundings, its temperature increases by,
A certain mass of an ideal diatomic gas contained in a closed vessel is heated. It is observed that half the amount of gas gets dissociated, but the temperature remains constant. The ratio of the heat supplied to the gas to the initial internal energy of the gas will be
An ideal refrigerator has a freezer at a temperature of . The coefficient of performance of the refrigerator is The temperature of the air (to which heat is rejected) in will be:
A carnot engine working between and has a work output of per cycle. The amount of heat energy supplied to the engine from the source in each cycle is
