Embibe Experts Solutions for Chapter: Structure of the Atom, Exercise 1: Exercise
Embibe Experts Science Solutions for Exercise - Embibe Experts Solutions for Chapter: Structure of the Atom, Exercise 1: Exercise
Attempt the practice questions on Chapter 4: Structure of the Atom, Exercise 1: Exercise with hints and solutions to strengthen your understanding. THINK ABOVE AND BEYOND SCIENCE PRACTICE BOOKS solutions are prepared by Experienced Embibe Experts.
Questions from Embibe Experts Solutions for Chapter: Structure of the Atom, Exercise 1: Exercise with Hints & Solutions
An element is a substance that cannot be broken down into simpler substances by chemical means. It is composed of atoms that have the same atomic number, that is, each atom has the same number of protons in its nucleus as all other atoms of that element.
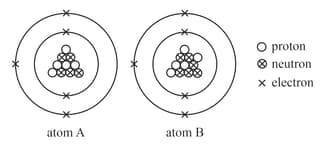
Raju is a th grade student, and his teacher taught him about elements and their subatomic particles. Numerous numerical representations are used while symbolising atoms. His teacher handed him a chart and instructed him to answer some questions based on the given chart. Will you assist Raju in answering the following questions?
- Could you tell which element the atoms represent and write their electronic configuration?
- What do we call such atoms together?
- Write their representation in the form of . Which one is heavier among atoms A and B?
Lithium is a metal that helps in anxiety, and the body's serotonin level works on its response. It is used for medications like anxiety and other mood disorders. It is a silvery-white metal with a symbol .
Lithium being an alkali metal is reactive and has the tendency to lose electrons. That makes it a good conductor. It has been widely used in EV automobiles in the form of Lithium-ion batteries.
On studying the atomic structure of Lithium on the basis of Bohr's postulate, a scientist illustrated a figure as follows.
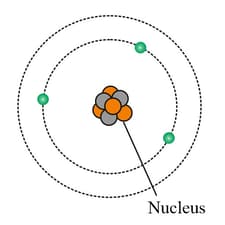
Could you Identify the error made by him and also explain the rings outside the nucleus?
Aarya studied the electronic configurations of some of the elements. He made a graph for the atomic number of the elements and their outer shell electrons. He plotted two different lines on his graph on the basis of the difference in their outer shell electrons. Experimentally he took one element from each of the groups and found that the element from series two shows an exothermic reaction with water while the element from series one does not react at all.
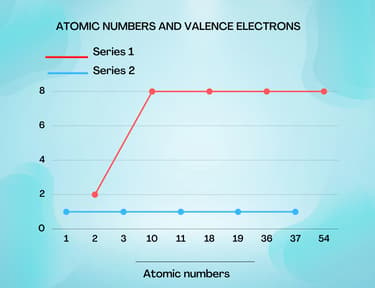
- Series one is not a straight line while series two has. Could you explain what difference it explains?
- What groups of elements do these series represent?
Myra and Anvesh were discussing Rutherford's Gold foil experiment. In the Rutherford Gold Foil experiment, a thin layer of gold is bombarded with minute particles. The gold-foil experiment showed that some of the particles were deflected far away from the center and some of them did not deflect at all. They passed in a straight line. Something dense has been assumed to be at the center of the atom while negatively charged electrons are assumed to be at a great distance from the center.
- What did Rutherford conclude from this experiment? Is the ray diagram given below correct?
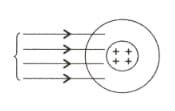
- Myra and Anvesh were asked to draw the complete ray diagram for this experiment?
Three forms of hydrogen are found in the atmosphere- Protium, Deuterium and Tritium, out of which protium is quite abundant, deuterium is said to be formed at the time of the Big Bang and tritium is highly radioactive. Tritium converts into helium through a beta decay.

Every atom when analysed has been found that they have only one orbit and only one electron revolves in that orbit. All of these have one proton in their nucleus as well.
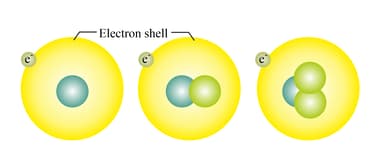
Could you identify the three different species from the picture given? What interference can you arrive at when the mass number and an atomic number of these elements are examined?
Element X is the second most abundant gas in the atmosphere. It is a colourless, odourless, tasteless gas. Element X is known as the elixir of life because, without this element, no one can survive on Earth. It also supports combustion. We get element X from the plant by the process of photosynthesis. Aquatic animals use this in the dissolved form in the water for respiration. The UV radiation splits the molecular element X apart into free X atoms. These atoms then combine with molecular oxygen to form ozone in the atmosphere. This ozone then shields us from the sun's harmful ultraviolet radiation (UV).
Element Y is a tasteless, odourless, bright yellow, crystalline solid at room temperature. It is a poor conductor of electricity and is insoluble in water. It also helps metabolise the food and protects your body from inflammation and oxidative stress. Element Y in the form of its oxides combines with water droplets in the air to make acid, which is part of acid rain. Acid rain is bad for plants, fish, and other living things.

In the below table, the atomic numbers, number of protons, electrons, and neutrons of elements X and Y are given. With the help of the below table, find out the names of the elements X and Y and the mass numbers of X and Y atoms.
|
Element |
Atomic Number |
Number of Protons |
Number of Neutrons |
Number of Electrons |
Distribution of Electrons
|
Valency |
|||
|
K |
L |
M |
N |
||||||
|
X |
8 |
8 |
8 |
8 |
2 |
6 |
- |
- |
2 |
|
Y |
16 |
16 |
16 |
16 |
2 |
8 |
6 |
- |
2 |
Element X is a shiny grey solid. It is naturally present in a variety of foods and is available as a supplement. It plays many crucial roles in the body, such as supporting muscle and nerve function and energy production. Element X burns bright white. Hence, it is used for making crackers to add white sparks and overall brilliance to a firework. An aqueous solution of element X acts as an antacid and is used to treat acidity produced in the stomach.
The ion of an element X has positive charge. The mass number of the element X is and the number of neutrons is . Write the name of the element X and What is the number of electrons in the ion?
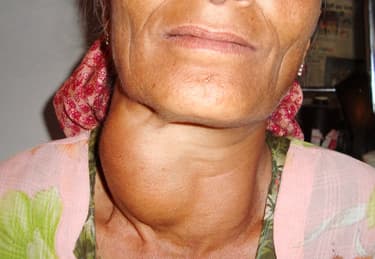
Sumana was not happy due to which she was unable to focus on her studies. Her friend Reena observed the same and enquired about Sumana. Sumana told Reena that her younger sister is also not in a good health. Her neck has swollen. The reason that came to light was that Sumana's family was not taking iodised salt in their diet. Reena suggested certain measures and insisted that Sumana's sister must consult the doctor immediately.
What can be the possible disease from which Suman's sister was suffering? Can you name the element whose isotope is used in the treatment of this disease?