Embibe Experts Solutions for Exercise 3: Assignment
Embibe Experts Chemistry Solutions for Exercise - Embibe Experts Solutions for Exercise 3: Assignment
Attempt the free practice questions from Exercise 3: Assignment with hints and solutions to strengthen your understanding. Gamma Question Bank for Medical Chemistry solutions are prepared by Experienced Embibe Experts.
Questions from Embibe Experts Solutions for Exercise 3: Assignment with Hints & Solutions
Enthalpy of formation of is and , are respectively and . The value of is
The heat of neutralisation for strong acid and strong base forming moles of water is
The value of in for the reaction will be if
The heat liberated on complete combustion of mole of gas to and is . Calculate the heat evolved by of on complete combustion.
The work done in an open vessel at , when iron reacts with dil to give , is nearly
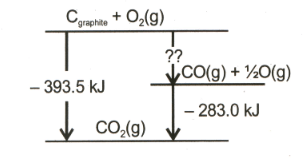
A schematic representation of enthalpy changes for The reaction, is given below. The missing value is
Which of the following equations respresents standard heat of formation of
Calorific value of ethane, in if for the reaction
