Gary Horner Solutions for Chapter: Form, Exercise 7: Data-based question: Cooking at altitude
Gary Horner Chemistry Solutions for Exercise - Gary Horner Solutions for Chapter: Form, Exercise 7: Data-based question: Cooking at altitude
Attempt the practice questions on Chapter 6: Form, Exercise 7: Data-based question: Cooking at altitude with hints and solutions to strengthen your understanding. MYP Chemistry A concept-based approach Years 4&5 solutions are prepared by Experienced Embibe Experts.
Questions from Gary Horner Solutions for Chapter: Form, Exercise 7: Data-based question: Cooking at altitude with Hints & Solutions
The boiling point of water is at an atmospheric pressure of atmosphere or , a typical level at sea level. At higher altitudes the air pressure decreases and the boiling point increases.
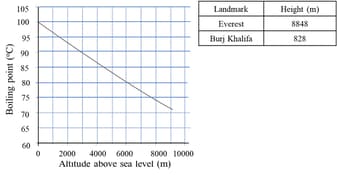
Study the graph and the table above. Use the information to calculate the percentage increase of the boiling point from the summit of Everest to the top of the Burj Khalifa.
The boiling point of water is at an atmospheric pressure of atmosphere or , a typical level at sea level. At higher altitudes the air pressure decreases and the boiling point increases.
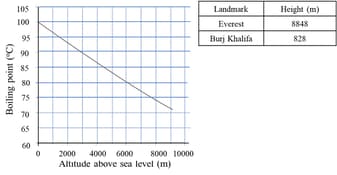
Will it take less time to cook pasta at the top Burj Khalifa or the summit of Everest? Explain your answer. What do you except to happen to the boiling point below sea level?
